
Introduction To Investigators Responsibilities With Good Clinical Practice | PDF | Institutional Review Board | Clinical Trial

ICH GCP - 8. Essential documents for the conduct of a clinical trial: ICH E6 (R2) Good clinical practice

PDF) The importance of Good Clinical Practice guidelines and its role in clinical trials | Anushya Vijayananthan - Academia.edu

Principles of Good Clinical Practice (GCP) – What is it all about and who is responsible for adherence? GCP and QA All SIAC Call Mar 14, 2008 Munish Mehra, - ppt download












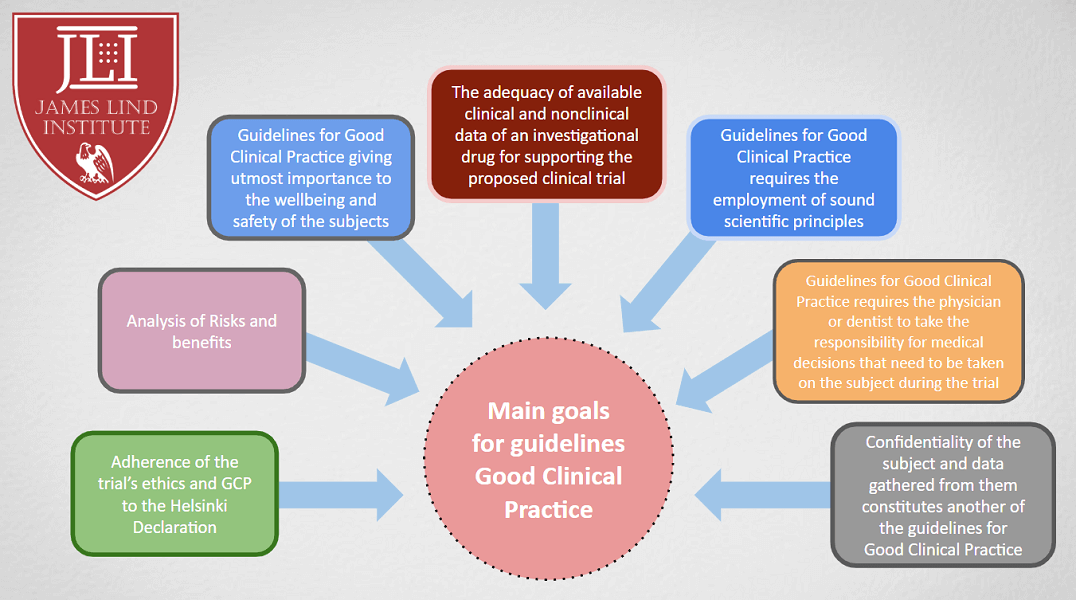





![Few Things [Nobody] Told You About Good Clinical Practice – Compliance4all Few Things [Nobody] Told You About Good Clinical Practice – Compliance4all](https://compliance4all14.files.wordpress.com/2018/09/good-clinical-practice-3-638.jpg)